

|
 |
|
| Silicate glass structure | Silicate crystalline structure | |
|
A Definition: The Structure of Glass Glass is not so much a material as it is a way of structuring material. What makes a glass a glass is the amorphous structural arrangement of its particles, regardless of chemical composition. Glass is often defined as a super-cooled liquid because the material has cooled from an amorphous liquid state at a rate too fast for the particles to break the amorphous bonds and form crystalline bonds typical of solids. Thus, glasses have a mechanical rigidity associated with solids and an amorphous structure associated with liquids. The Structure of Transparency: The hybrid liquid/solid of glass creates a material with unique optical properties. Transparency to visible light is a factor of two qualities; the lack of internal reflective surfaces which reflect and trap light and the absence of free electrons which absorb photons of light to balance out their electrical charge. The boundaries of a crystalline structure create internal reflective surfaces. In glasses and liquids boundaries between particles fuse, allowing for the transmission of light. The absence or presence of free electrons is an attribute of composition and bonding relationships. Transparent glasses are formed from the ionic bonding of elements to create a neutral charge that is not interested in absorbing and trapping light photons. Varying degrees of transparency can be engineered through structure (devitrification), composition or surface flaws (acid or abrasion). Silicate Glasses: The glasses we most commonly produce are silicate based. This is due to The abundance of silica in the Earth's crust and the fact that silica readily cools in an amorphous state under ambient and or typical factory conditions. The bonds formed between silicon and oxygen in SiO44- tetrahedra, the building blocks of all silicate rocks and minerals including glass, are exceptionally strong. In the transition from a liquid state the strong tetrahedral bonds within the amorphous structure must break before they can rejoin in a uniformly crystalline structure. This cumbersome process takes a relatively long time and so only silicates with rates of cooling sufficiently slowed down, by conditions such as insulated plutons of igneous rock within the Earth and kilns which can be programed to hold transition temperatures, form crystalline materials. |
||
|
Workability
and Viscosity Silicate glass does not have a melting point. It goes through gradual increases and decreases of viscosity with temperature change; this is the key to understanding the glassmaker's art. Essentially glass acts as a different material with distinct qualities at different temperatures. At high temperatures glass is viscous to viscoelastic, moving like honey on the end of the glassblower's pipe. The degree of viscous flow at the time of forming is recorded in the memory of the glass. At lower temperatures glass becomes linearly elastic. At room temperatures silicate glasses are perfectly elastic; they will not permanently deform but rather will always return to their original position until reaching a point of fracture. At low temperatures glass is rigid and typically brittle. It can be cut, ground and polished. The mid ranged temperatures in glass forming can be particularly interesting, offering a degree of control that could be very useful at an architectural scale. Having a leather-soft consistency, glass can be readily bent in two or three dimensions and cut easily with shears. |
||
|
Properties and
Phenomena Glass can be engineered to excel in virtually any area of performance phenomena. The two key factors in engineering a glass to have particular attributes are time/temperature rates and the chemical bonding of elements to form alloys. Time/temperature rates have already been touched on in the previous section on workability and viscosity. Alloys are combinations of elements which produce a material with qualities that the individual constituents did not have on their own. Alloys are always stronger than their individual elements. Glasses are currently engineered for a multitude of high performance phenomena ranging from supper conductors to very good electrical, thermal and acoustical insulators. Typically, glass alloys are formed by modifying a silicate network with a modifier, as in borosilicate glass where alkali oxides are replaced by boric oxides to reduce the thermal expansion of the glass. Recently, a variety of glass metal composites have been produced and exhibit unique properties of transparency combined with hardness and ductility. Due to the nature of high atomic bonding in silicates, the theoretical strengths of silicate glasses are very high but the weibull modulus of material predictability is of variable consistency and so this consistency of glass strength is an area of intense research in materials science. Two current approaches to maximizing the available strength of glasses are to induce compressive surface stresses or to use bundles of small fibers. Materials in their fiber phase, particularly brittle materials such as glass, are stronger than material in bulk form. This is due to the reduced probability of surface flaw with decreasing specimen volume. Thermal or chemical toughening is commonly used to induce surface stresses in window glass. An approach to thermal toughening in situ is discussed in the design section of this report. |
||
|
Weathering Weathering can often be a graceful evolution of a buildings form and materiality. The design and construction phase of a building is mainly a strategic beginning. An understanding of the environment and its effect on the materials used allows for a conscious engagement of design with the life and forces which will continue to shape a building. The making of a glass architecture in situ begins with a conscious engagement of these forces in the landscape and so a continuity of this mindset is natural. Abrasion Chemical Exposure Light |
||
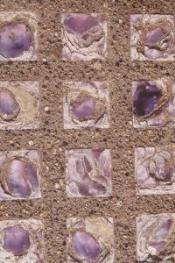 |
Glass Sidewalk pavers that have turned shades of transparent purple with exposure to sunlight over the years. Manganese was once used as a decolourant in glass to counter the green tint of iron oxides which are commonly present in silica deposits. It was only discovered with the passing of time that the manganese has a slow, gradual reaction to Ultra Violet light causing it to take on a rosy blush which deepens over time with continued exposure. The observation of this accidental photochemical reaction lead to developments in photography and photo chemical etching processes for computer chips. |